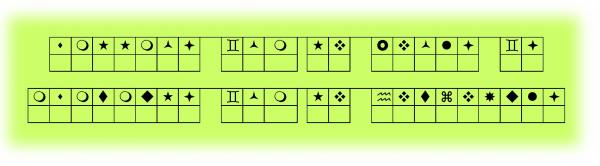
Engage: Mystery Message
Can you interpret the message below?
Do you need some help? The following tool should help you to decode the mystery message.
Write the decoded message in your notebook. Or, print a copy of the "Mystery Message" handout to complete the decoding activity. The handout is located in Related Items.
Explore: Getting to Know You
Get reacquainted with elements and learn about compounds!
Click on the video box below to watch the video.
As you watch the video, listen for
- Descriptions/characteristics of elements
- Descriptions/characteristics of compounds
- Examples of elements
- Examples of compounds
Record your findings in your science journal.
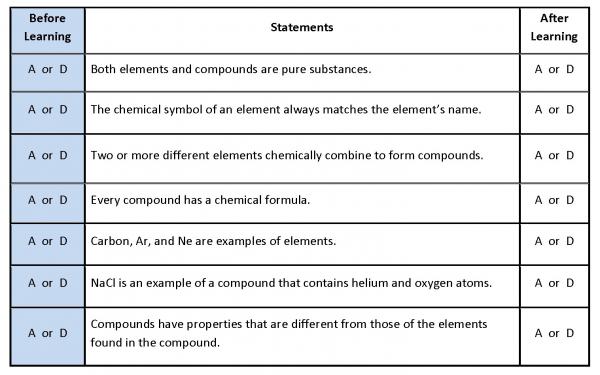
Explain 1: Anticipation Guide
It’s time to learn more about elements and compounds. But first, let’s look at what you might already know.
Print a copy of the "Anticipation Guide" found in Related Items. Read each of the statements in the anticipation guide. If you agree with the statement, circle A in the Before Learning column. If you disagree with the statement, circle D in the Before Learning column.
Explain 2: Elements and Compounds
Once elements chemically combine to form compounds, it is hard to separate the elements out of the compound. Recall that mixtures are substances that have been physically joined together and are usually easily separated by physical means.
Click between the two dark lines below to learn more about the differences between elements and compounds. Don’t forget to add notes in your journal!
After completing the video, scroll down for instructions to complete the "Anticipation Guide."
Revisit the "Anticipation Guide" by rereading each statement and circling A for agree or D for disagree in the right column labeled After Learning.
An answer key can be found in Related Items.
Explain 3: Characteristics of Elements and Compounds
Can you identify characteristics of an element and a compound?
Complete the following activity by identifying each phrase as describing either an element or a compound. To retake the quiz, reload the page and then select No when the Resume Quiz dialog box appears.
Elaborate 1a: Decoding Symbols and Formulas
The Periodic Table is a tool that helps scientists determine elements found in compounds. You could think of the Periodic Table as a decoder, similar to the one used in Engage.
Click on the white video box below to learn how to use the Periodic Table as a decoder for elements and compounds. You are encouraged to stop the video to work the problems and to replay the information.
A Periodic Table is needed for both the video. Look in Related Items for a downloadable Periodic Table.
Elaborate 1b: Decoding Symbols and Formulas
Click below to begin the Decoding Compounds activity. To retake the quiz, reload the page and then select No when the Resume Quiz dialog box appears. A Periodic Table is needed for the activity. Look in Related Items for a downloadable Periodic Table
Elaborate 2: Decoding Table and Periodic Table
How is the Periodic Table similar to the decoding tool you used in the Engage?
Evaluate: Assess Your Learning!
Teacher Notes
In this lesson, students differentiate between elements and compounds on the most basic level. Students will learn the basic characteristics of elements and compounds. While students are introduced to molecule and atoms in the videos, these terms are not explored in this lesson. Molecule is introduced in the seventh grade TEKS (7)(6)(C), and atoms and subscripts are introduced in the eighth grade TEKS (8)(5)(D). Avoid the misconception that only compounds are represented by chemical formulas. Elemental molecules such as O2, Cl2, O3, etc., are also represented by chemical formulas. Since this TEKS addresses elements and compounds at the most basic level, it is inappropriate (and confusing to students) to include this concept with most sixth-grade students.
| Lesson Cycle | Lesson Activities and Notes |
|---|---|
|
Engage Mystery Message |
Students engage in decoding a message. Later in the lesson, students will compare the decoder tool to the Periodic Table. The decoded message should read “Letters are to words as elements are to compounds.” Students will be asked to explain this analogy later in the lesson. Throughout this lesson, the Periodic Table is shown as a tool to help “decode” compounds and elements. |
|
Explore Getting the Know You |
Student are introduced to and given examples of elements and compounds through the video They Must Be Giants—Meet the Elements. |
|
Explain 1 Anticipation Guide |
Students begin the Explain by completing the Before Learning section of the Anticipation Guide. The Anticipation Guide helps students focus on the important differences between elements and compounds. |
|
Explain 2 Elements and Compounds |
Students watch a video that explains the differences between elements and compounds. Teachers are encouraged to help students understand the differences between compounds (chemically combined) and mixtures (physically combined). Mixtures are included in the grade 3, 4, and 5 TEKS. The terms atoms and molecules are introduced to students. Students should be encouraged to take notes in their journals. Students revisiting the anticipation guide and complete the After Learning column. The anticipation guide and an answer key are available in Related Items. |
|
Explain 3 Characteristics of Elements and Compounds |
Students work through an interactive card sort, sorting element and compound descriptors. |
|
Elaborate 1 Decoding Symbols and Chemical Formulas |
Students watch a video that models decoding the elements found in compounds and introduces the use of models to represent elements and compounds. Students are shown that subscripts represent atoms of each element, but this is not the focus of the lesson, nor should students determine the number of atoms in a compound in sixth grade. After viewing the video, students complete an interactive activity to determine the number of elements in different compounds and to identify the elements found in the compound. Students should use their Periodic Table during both the video and activity. |
|
Elaborate 2 Decoding Table and Periodic Table |
Students are asked to recall the Engage activity and relate it to the Periodic Table. Students should consider compounds are made up of a variety of elements, just like words are made of letters. There are simple and complex words, just like there are simple and complex compounds. The number of letters in words varies, just like the number of elements in a compound varies. Letters found in a word can be rearranged to form a new word. The same is true for elements and compounds. |
|
Evaluate Assessing Your Learning |
Students assess their learning using several multiple choice questions and a Venn Diagram. |