Learning Objectives
Learning Objectives
In this section, you will explore the following questions:
- How does the fluid mosaic model describe the structure and components of the plasma cell membrane?
- How do the molecular components of the membrane provide fluidity?
Connection for AP® Courses
Connection for AP® Courses
Like an art mosaic, the plasma membrane consists of several different components. Phospholipids (which we studied in previously) form a bilayer; the hydrophobic, fatty acid tails are in contact with each other, and hydrophilic portions of the phospholipids are oriented toward the aqueous internal and external environments. Several types of proteins with different functions stud the membrane. Integral proteins often span the membrane and can transport materials into or out of the cells; these embedded proteins can be hydrophilic or hydrophobic, depending on their placement within the membrane. Peripheral proteins found on the exterior and interior surfaces of membranes can serve as enzymes, structural attachments for fibers of the cytoskeleton, and part of a cell’s recognition sites. These cell-specific proteins play a vital role in immune function; they enable cells of a certain type (e.g., liver cells) to identify each other when forming a tissue, and allow hormones and other molecules to recognize target cells. These proteins float throughout the membrane, constantly in flux.
Information presented and the examples highlighted in this section support concepts and learning objectives outlined in Big Idea 2 of the AP® Biology Curriculum Framework. The learning objectives provide a transparent foundation for the AP® Biology course, an inquiry-based laboratory experience, instructional activities, and AP® exam questions. A learning objective merges required content with one or more of the seven science practices(SPs).
| Big Idea 2 | Biological systems use free energy and molecular building blocks to grow, reproduce, and to maintain dynamic homeostasis. |
| Enduring Understanding 2.B | Growth, reproduction, and dynamic homeostasis require that cells create and maintain internal environments that are different from their external environments. |
| Essential Knowledge | 2.B.1 Cell membranes are selectively permeable due to their structure. |
| Science Practice | 1.4 The student is able to use representations and models to analyze situations or solve problems qualitatively and quantitatively. |
| Science Practice | 3.1 The student can pose scientific questions. |
| Learning Objective | 2.10 The student is able to use representations and models to pose scientific questions about the properties of cell membranes and selective permeability based on molecular structure. |
| Essential Knowledge | 2.B.1 Cell membranes are selectively permeable due to their structure. |
| Science Practice | 1.1 The student can create representations and models of natural or man-made phenomena and systems in the domain. |
| Science Practice | 7.1 The student can connect phenomena and models across spatial and temporal scales. |
| Science Practice | 7.2 The student can connect concepts in and across domain(s) to generalize and extrapolate in and/or across enduring understandings and/or big ideas. |
| Learning Objective | 2.11 The student is able to construct models that connect the movement of molecules across a membrane with membrane structure and function. |
In addition, content from this chapter is addressed in the AP Biology Laboratory Manual in the following lab(s):
- 6 Diffusion and Osmosis
A cell’s plasma membrane defines the cell, outlines its borders, and determines the nature of its interaction with its environment (see Figure 5.2 for a summary). Cells exclude some substances, take in others, and excrete still others, all in controlled quantities. The plasma membrane must be very flexible to allow certain cells, such as red blood cells and white blood cells, to change shape as they pass through narrow capillaries. These are the more obvious functions of a plasma membrane. In addition, the surface of the plasma membrane carries markers that allow cells to recognize one another, which is vital for tissue and organ formation during early development, and which later plays a role in the self versus nonself distinction of the immune response.
Among the most sophisticated functions of the plasma membrane is the ability to transmit signals by means of complex, integral proteins known as receptors. These proteins act both as receivers of extracellular inputs and as activators of intracellular processes. These membrane receptors provide extracellular attachment sites for effectors such as hormones and growth factors, and they activate intracellular response cascades when their effectors are bound. Occasionally, receptors are hijacked by viruses that use them to gain entry into cells, and at times, the genes encoding the receptors become mutated, causing the process of signal transduction to malfunction with disastrous consequences.
Fluid Mosaic Model
Fluid Mosaic Model
The existence of the plasma membrane was identified in the 1890s, and its chemical components were identified in 1915. The principal components identified at that time were lipids and proteins. The first widely accepted model of the plasma membrane’s structure was proposed in 1935 by Hugh Davson and James Danielli, based on the railroad track appearance of the plasma membrane in early electron micrographs. They theorized that the structure of the plasma membrane resembled a sandwich, with protein being analogous to the bread, and lipids being analogous to the filling. In the 1950s, advances in microscopy, notably transmission electron microscopy (TEM), allowed researchers to see that the core of the plasma membrane consisted of a double, rather than a single, layer. A new model that better explains both the microscopic observations and the function of that plasma membrane was proposed by S.J. Singer and Garth L. Nicolson in 1972.
The explanation proposed by Singer and Nicolson is called the fluid mosaic model. The model has evolved somewhat over time, but it still best accounts for the structure and functions of the plasma membrane as we now understand them. The fluid mosaic model describes the structure of the plasma membrane as a mosaic of components, including phospholipids, cholesterol, proteins, and carbohydrates, that gives the membrane a fluid character. Plasma membranes range from five to 10 nm in thickness. For comparison, human red blood cells, visible via light microscopy, are approximately eight µm wide, or approximately 1,000 times wider than a plasma membrane. The membrane does look a bit like a sandwich (Figure 5.2).
The principal components of a plasma membrane are lipids (phospholipids and cholesterol), proteins, and carbohydrates attached to some of the lipids and some of the proteins. A phospholipid is a molecule consisting of glycerol, two fatty acids, and a phosphate-linked head group. Cholesterol, another lipid composed of four fused carbon rings, is found alongside the phospholipids in the core of the membrane. The proportions of proteins, lipids, and carbohydrates in the plasma membrane vary with cell type. For a typical human cell, protein accounts for about 50 percent of the composition by mass, lipids (of all types) account for about 40 percent of the composition by mass, and carbohydrates complete the remaining 10 percent of the composition by mass. However, the concentration of proteins and lipids varies with different cell membranes. For example, myelin, an outgrowth of the membrane of specialized cells that insulates the axons of the peripheral nerves, contains only 18 percent proteins and 76 percent lipids. The mitochondrial inner membrane contains 76 percent proteins and only 24 percent lipids. The plasma membrane of human red blood cells is 30 percent lipids. Carbohydrates are present only on the exterior surface of the plasma membrane and are attached to proteins, forming glycoproteins, or attached to lipids, forming glycolipids.
Phospholipids
The main fabric of the membrane is composed of amphiphilic, phospholipid molecules. The hydrophilic or water-loving areas of these molecules, which look like a collection of balls in an artist’s rendition of the model in Figure 5.2, are in contact with the aqueous fluid both inside and outside the cell. Hydrophobic, or water-hating molecules, tend to be nonpolar. They interact with other nonpolar molecules in chemical reactions, but generally do not interact with polar molecules. When placed in water, hydrophobic molecules tend to form a ball or cluster. The hydrophilic regions of the phospholipids tend to form hydrogen bonds with water and other polar molecules on both the exterior and interior of the cell. Thus, the membrane surfaces that face the interior and exterior of the cell are hydrophilic. In contrast, the interior of the cell membrane is hydrophobic and will not interact with water. Therefore, phospholipids form an excellent two-layer cell membrane that separates fluid within the cell from the fluid outside the cell.
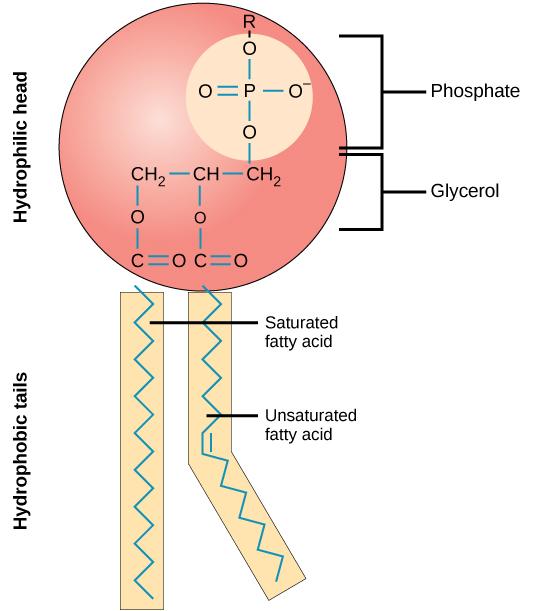
A phospholipid molecule (Figure 5.3) consists of a three-carbon glycerol backbone with two fatty acid molecules attached to carbons one and two, and a phosphate-containing group attached to the third carbon. This arrangement gives the overall molecule an area described as its head (the phosphate-containing group), which has a polar character or negative charge, and an area called the tail (the fatty acids), which has no charge. The head can form hydrogen bonds, but the tail cannot. A molecule with this arrangement of a positively or negatively charged area and an uncharged, or nonpolar, area is referred to as amphiphilic or dual-loving.
This characteristic is vital to the structure of a plasma membrane because in water, phospholipids tend to become arranged with their hydrophobic tails facing each other and their hydrophilic heads facing out. In this way, they form a lipid bilayer—a barrier composed of a double layer of phospholipids that separates the water and other materials on one side of the barrier from the water, and other materials on the other side. In fact, phospholipids heated in an aqueous solution tend to spontaneously form small spheres or droplets, called micelles or liposomes, with their hydrophilic heads forming the exterior and their hydrophobic tails on the inside (Figure 5.4).
Proteins
Proteins make up the second major component of plasma membranes. Integral proteins (some specialized types are called integrins) are, as their name suggests, integrated completely into membrane structure, and their hydrophobic membrane-spanning regions interact with the hydrophobic region of the the phospholipid bilayer (see Figure 5.2). Single-pass integral membrane proteins usually have a hydrophobic transmembrane segment that consists of 20–25 amino acids. Some span only part of the membrane associating with a single layer while others stretch from one side of the membrane to the others and are exposed on either side. Some complex proteins are composed of up to 12 segments of a single protein, which are extensively folded and embedded in the membrane (Figure 5.5). This type of protein has a hydrophilic region or regions, and one or several mildly hydrophobic regions. This arrangement of regions of the protein tends to orient the protein alongside the phospholipids, with the hydrophobic region of the protein adjacent to the tails of the phospholipids, and the hydrophilic region or regions of the protein protruding from the membrane and in contact with the cytosol or extracellular fluid.
Peripheral proteins are found on the exterior and interior surfaces of membranes, attached either to integral proteins or to phospholipids. Peripheral proteins, along with integral proteins, may serve as enzymes, as structural attachments for the fibers of the cytoskeleton, or as part of the cell’s recognition sites. These are sometimes referred to as cell-specific proteins. The body recognizes its own proteins and attacks foreign proteins associated with invasive pathogens.
Carbohydrates
Carbohydrates are the third major component of plasma membranes. They are always found on the exterior surface of cells and are bound either to proteins (forming glycoproteins) or to lipids (forming glycolipids) as seen in Figure 5.2. These carbohydrate chains may consist of two to 60 monosaccharide units and can be either straight or branched. Along with peripheral proteins, carbohydrates form specialized sites on the cell surface that allow cells to recognize each other. These sites have unique patterns that allow the cell to be recognized, much the same way that the facial features unique to each person allow him or her to be recognized. This recognition function is very important to cells, as it allows the same immune system to differentiate between body cells, called self, and foreign cells or tissues, called nonself. Similar types of glycoproteins and glycolipids are found on the surfaces of viruses and may change frequently, preventing immune cells from recognizing and attacking them.
These carbohydrates on the exterior surface of the cell—the carbohydrate components of both glycoproteins and glycolipids—are collectively referred to as the glycocalyx, meaning sugar coating. The glycocalyx is highly hydrophilic and attracts large amounts of water to the surface of the cell. This aids in the interaction of the cell with its watery environment and in the cell’s ability to obtain substances dissolved in the water. As discussed above, the glycocalyx is also important for cell identification, self/nonself determination, and embryonic development, and is used in cell–cell attachments to form tissues.
Evolution Connection
How Viruses Infect Specific Organs
Glycoprotein and glycolipid patterns on the surfaces of cells give many viruses an opportunity for infection. The human immunodeficiency virus (HIV) and hepatitis virus infect only specific organs or cells in the human body. HIV is able to penetrate the plasma membranes of a subtype of lymphocytes called T-helper cells, as well as some monocytes and central nervous system cells. The hepatitis virus attacks liver cells.
These viruses are able to invade these cells because the cells have binding sites on their surfaces that are specific to and compatible with certain viruses (Figure 5.6). Other recognition sites on the virus’s surface interact with the human immune system, prompting the body to produce antibodies. Antibodies are made in response to the antigens or proteins associated with invasive pathogens, or in response to foreign cells, such as might occur with an organ transplant. These same sites serve as places for antibodies to attach and either destroy or inhibit the activity of the virus. Unfortunately, these recognition sites on HIV change at a rapid rate because of mutations, making the production of an effective vaccine against the virus very difficult, as the virus evolves and adapts. A person infected with HIV will quickly develop different populations, or variants, of the virus that are distinguished by differences in these recognition sites. This rapid change of surface markers decreases the effectiveness of the person’s immune system in attacking the virus, because the antibodies will not recognize the new variations of the surface patterns. In the case of HIV, the problem is compounded by the fact that the virus specifically infects and destroys cells involved in the immune response, further incapacitating the host.
Why does the immune system attack a transplanted organ?
- Glycoproteins and glycolipids on the surface of the organ’s cells are similar to those found on pathogens.
- Glycoproteins and glycolipids on the surface of the organ’s cells are not recognized by the immune system.
- Glycoproteins and glycolipids on the surface of the organ’s cells are toxic to the body.
- Glycoproteins and glycolipids on the surface of the organ’s cells are similar to those found on immune cells.
Membrane Fluidity
Membrane Fluidity
The mosaic characteristic of the membrane, described in the fluid mosaic model, helps to illustrate its nature. The integral proteins and lipids exist in the membrane as separate but loosely attached molecules. These resemble the separate, multicolored tiles of a mosaic picture, and they float, moving somewhat with respect to one another. However, the membrane is not like a balloon that can expand and contract; rather, it is fairly rigid and can burst if penetrated or if a cell takes in too much water. Because of its mosaic nature, a very fine needle can easily penetrate a plasma membrane without causing it to burst, and the membrane will flow and self-seal when the needle is extracted.
The mosaic characteristics of the membrane explain some but not all of its fluidity. There are two other factors that help maintain this fluid characteristic. One factor is the nature of the phospholipids themselves. In their saturated form, the fatty acids in phospholipid tails are saturated with bound hydrogen atoms. There are no double bonds between adjacent carbon atoms. This results in tails that are relatively straight. In contrast, unsaturated fatty acids do not contain a maximal number of hydrogen atoms, but they do contain some double bonds between adjacent carbon atoms; A double bond results in a bend in the string of carbons of approximately 30° (see Figure 5.3).
Thus, if saturated fatty acids, with their straight tails, are compressed by decreasing temperatures, they press in on each other, making a dense and fairly rigid membrane. If unsaturated fatty acids are compressed, the kinks in their tails nudge adjacent phospholipid molecules away, maintaining some space between the phospholipid molecules. This extra helps to maintain fluidity in the membrane at temperatures at which membranes with saturated fatty acid tails in their phospholipids would freeze or solidify. The relative fluidity of the membrane is particularly important in a cold environment. A cold environment tends to compress membranes composed largely of saturated fatty acids, making them less fluid and more susceptible to rupturing. Many organisms (fish are one example) are capable of adapting to cold environments by changing the proportion of unsaturated fatty acids in their membranes in response to the lowering of the temperature.
Link to Learning
Visit this site to see animations of the fluidity and mosaic quality of membranes.
Explain why glucose cannot pass directly through the cell membrane.
- The plasma membrane is impermeable to polar molecules, so transport proteins are required.
- The plasma membrane is selectively permeable to polar molecules, and a transport protein is required for larger molecules.
- The plasma membrane is permeable to all polar molecules, but a transport protein is required.
- The plasma membrane is selectively permeable to all polar molecules, and a transport protein is never required for them.
Animals have an additional membrane constituent that assists in maintaining fluidity. Cholesterol, which lies alongside the phospholipids in the membrane, tends to dampen the effects of temperature on the membrane. This lipid functions as a buffer, preventing lower temperatures from inhibiting fluidity, and preventing increased temperatures from increasing fluidity too much. Thus, cholesterol extends, in both directions, the temperature range in which the membrane is appropriately fluid and consequently functional. Cholesterol also serves other functions, such as organizing clusters of transmembrane proteins into lipid rafts.
| The Components and Functions of the Plasma Membrane | |
|---|---|
| Component | Location |
| Phospholipid | Main fabric of the membrane |
| Cholesterol | Attached between phospholipids and between the two phospholipid layers |
| Integral proteins (e.g., integrins) | Embedded within the phospholipid layer(s); may or may not penetrate through both layers |
| Peripheral proteins | On the inner or outer surface of the phospholipid bilayer; not embedded within the phospholipids |
| Carbohydrates (components of glycoproteins and glycolipids) | Generally attached to proteins on the outside membrane layer |
Career Connection
Immunologist
The variations in peripheral proteins and carbohydrates that affect a cell’s recognition sites are of prime interest in immunology. These changes are taken into consideration in vaccine development. Many infectious diseases, such as smallpox, polio, diphtheria, and tetanus, were conquered by the use of vaccines.
Immunologists are the physicians and scientists who research and develop vaccines, as well as treat and study allergies or other immune problems. Some immunologists study and treat autoimmune diseases in which a person’s immune system attacks his or her own cells or tissues, such as lupus, and immunodeficiencies, whether acquired by a virus or hereditary (such as severe combined immunodeficiency, or SCID). Immunologists are called in to help treat organ transplantation patients, who must have their immune systems suppressed so that their bodies will not reject a transplanted organ. Some immunologists work to understand natural immunity and the effects of a person’s environment on it. Others work on questions about how the immune system affects the development of certain chronic diseases.
To work as an immunologist, a PhD or MD is required. In addition, immunologists undertake at least two to three years of training in an accredited program and must pass an examination given by the American Board of Allergy and Immunology. Immunologists must possess knowledge of the functions of the human body as they relate to issues beyond immunization, and knowledge of pharmacology and medical technology, such as medications, therapies, test materials, and surgical procedures.
Science Practice Connection for AP® Courses
Activity
Using appropriate media, construct a model of the plasma membrane and its molecular components. In the next section, you will use the model to demonstrate the movement of different substances across the membrane.
Think About It
What research questions can be asked about plasma membranes? State three questions relating to plasma membranes, along with possible solutions to the questions.
Short Answer
Short Answer
According to the fluid mosaic model of the plasma cell membrane, describe at least three primary functions of carbohydrates attached to the exterior of cell membranes.
Which characteristics of a phospholipid contribute to the fluidity of the cell membrane? Explain your answer.
Disclaimer
This section may include links to websites that contain links to articles on unrelated topics. See the preface for more information.