Learning Objectives
Learning Objectives
In this section, you will investigate the following questions:
- Why is carbon important for life?
- How do functional groups determine the properties of biological molecules?
Connection for AP® Courses
Connection for AP® Courses
The unique properties of carbon make it a central part of biological molecules. With four valence electrons, carbon can covalently bond to oxygen, hydrogen, and nitrogen to form the many molecules important for cellular function. Carbon and hydrogen can form either hydrocarbon chains or rings. Functional groups, such as –CH3 (methyl) and –COOH (carboxyl), are groups of atoms that give specific properties to hydrocarbon chains or rings that define their overall chemical characteristics and function. For example, the attachment of a carboxyl group (-COOH) makes a molecule more acidic, whereas the presence of an amine group (NH2) makes a molecule more basic. As we will explore in the next chapter, amino acids have both a carboxyl group and an amine group. Isomers are molecules with the same molecular formula (i.e., same kinds and numbers of atoms), but different molecular structures resulting in different properties or functions. (Don’t confuse isomer with isotope!)
The information presented and examples highlighted in this section support concepts and Learning Objectives outlined in Big Idea 2 of the AP® Biology Curriculum Framework. The Learning Objectives listed in the Curriculum Framework provide a transparent foundation for the AP® Biology course, an inquiry-based laboratory experience, instructional activities, and AP® Exam questions. A Learning Objective merges required content with one or more of the seven Science Practices.
| Big Idea 2 | Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. |
| Enduring Understanding 2.A | Growth, reproduction, and maintenance of living systems require free energy and matter. |
| Essential Knowledge | 2.A.3 Organisms must exchange matter with the environment to grow, reproduce and maintain organization. |
| Science Practice | 4.1 The student can justify the selection of the kind of data needed to answer a particular scientific question. |
| Learning Objective | 2.8 The student is able to justify the selection of data regarding the types of molecules that an animal, plant, or bacterium will take up as necessary building blocks and excrete as waste products. |
Cells are made of many complex molecules called macromolecules, such as proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids. The macromolecules are a subset of organic molecules, any carbon-containing liquid, solid, or gas that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or backbone, of the macromolecules.
Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: It has the chemical formula CH4. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell.
Hydrocarbons
Hydrocarbons
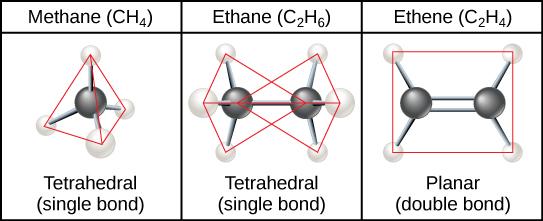
Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane (CH4) described above. We often use hydrocarbons in our daily lives as fuels—like the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which is released when these molecules are burned (oxidized). Methane, an excellent fuel, is the simplest hydrocarbon molecule, with a central carbon atom bonded to four different hydrogen atoms, as illustrated in Figure 2.24. The geometry of the methane molecule, where the atoms reside in three dimensions, is determined by the shape of its electron orbitals. The carbons and the four hydrogen atoms form a shape known as a tetrahedron, with four triangular faces; for this reason, methane is described as having tetrahedral geometry.
As the backbone of the large molecules of living things, hydrocarbons may exist as linear carbon chains, carbon rings, or combinations of both. Furthermore, individual carbon-to-carbon bonds may be single, double, or triple covalent bonds, and each type of bond affects the geometry of the molecule in a specific way. This three-dimensional shape or conformation of the large molecules of life (macromolecules) is critical to how they function.
Hydrocarbon Chains
Hydrocarbon chains are formed by successive bonds between carbon atoms and may be branched or unbranched. Furthermore, the overall geometry of the molecule is altered by the different geometries of single, double, and triple covalent bonds, illustrated in Figure 2.25. The hydrocarbons ethane, ethene, and ethyne serve as examples of how different carbon-to-carbon bonds affect the geometry of the molecule. The names of all three molecules start with the prefix “eth-,” which is the prefix for two carbon hydrocarbons. The suffixes “-ane,” “-ene,” and “-yne” refer to the presence of single, double, or triple carbon-carbon bonds, respectively. Thus, propane, propene, and propyne follow the same pattern with three carbon molecules, butane, butane, and butyne for four carbon molecules, and so on. Double and triple bonds change the geometry of the molecule: Single bonds allow rotation along the axis of the bond, whereas double bonds lead to a planar configuration and triple bonds to a linear one. These geometries have a significant impact on the shape a particular molecule can assume.
Hydrocarbon Rings
So far, the hydrocarbons we have discussed have been aliphatic hydrocarbons, which consist of linear chains of carbon atoms. Another type of hydrocarbon, aromatic hydrocarbons, consists of closed rings of carbon atoms. Ring structures are found in hydrocarbons, sometimes with the presence of double bonds, which can be seen by comparing the structure of cyclohexane to benzene in Figure 2.26. Examples of biological molecules that incorporate the benzene ring include some amino acids and cholesterol and its derivatives, including the hormones estrogen and testosterone. The benzene ring is also found in the herbicide 2,4-D. Benzene is a natural component of crude oil and has been classified as a carcinogen. Some hydrocarbons have both aliphatic and aromatic portions; beta-carotene is an example of such a hydrocarbon.
Isomers
Isomers
The three-dimensional placement of atoms and chemical bonds within organic molecules is central to understanding their chemistry. Molecules that share the same chemical formula but differ in the placement (structure) of their atoms and/or chemical bonds are known as isomers. Structural isomers (like butane and isobutene shown in Figure 2.27a) differ in the placement of their covalent bonds: Both molecules have four carbons and ten hydrogens (C4H10), but the different arrangement of the atoms within the molecules leads to differences in their chemical properties. For example, due to their different chemical properties, butane is suited for use as a fuel for torches, whereas isobutene is suited for use as a refrigerant and a propellant in spray cans.
Geometric isomers, on the other hand, have similar placements of their covalent bonds but differ in how these bonds are made to the surrounding atoms, especially in carbon-to-carbon double bonds. In the simple molecule butene (C4H8), the two methyl groups (CH3) can be on either side of the double covalent bond central to the molecule, as illustrated in Figure 2.27b. When the carbons are bound on the same side of the double bond, this is the cis configuration; if they are on opposite sides of the double bond, it is a trans configuration. In the trans configuration, the carbons form a more or less linear structure, whereas the carbons in the cis configuration make a bend, change in direction, of the carbon backbone.
Visual Connection
- Molecules with the formulas and could be structural isomers.
- Molecules must have a double bond to be cis-trans isomers.
- To be enantiomers, a molecule must have at least three different atoms or groups connected to a central carbon.
- To be enantiomers, a molecule must have at least four different atoms or groups connected to a central carbon.
In triglycerides (fats and oils), long carbon chains known as fatty acids may contain double bonds, which can be in either the cis or trans configuration, illustrated in Figure 2.28. Fats with at least one double bond between carbon atoms are unsaturated fats. When some of these bonds are in the cis configuration, the resulting bend in the carbon backbone of the chain means that triglyceride molecules cannot pack tightly, so they remain liquid (oil) at room temperature. On the other hand, triglycerides with trans double bonds, popularly called trans fats, have relatively linear fatty acids that are able to pack tightly together at room temperature and form solid fats. In the human diet, trans fats are linked to an increased risk of cardiovascular disease, so many food manufacturers have reduced or eliminated their use in recent years. In contrast to unsaturated fats, triglycerides without double bonds between carbon atoms are called saturated fats, meaning that they contain all the hydrogen atoms available. Saturated fats are a solid at room temperature and usually of animal origin.
Enantiomers
Enantiomers
Enantiomers are molecules that share the same chemical structure and chemical bonds but differ in the three-dimensional placement of atoms so that they are mirror images. As shown in Figure 2.29, an amino acid alanine example, the two structures are non-superimposable. In nature, only the L-forms of amino acids are used to make proteins. Some D forms of amino acids are seen in the cell walls of bacteria, but never in their proteins. Similarly, the D-form of glucose is the main product of photosynthesis and the L-form of the molecule is rarely seen in nature.
Functional Groups
Functional Groups
Functional groups are groups of atoms that occur within molecules and confer specific chemical properties to those molecules. They are found along the carbon backbone of macromolecules. This carbon backbone is formed by chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen. Molecules with other elements in their carbon backbone are substituted hydrocarbons.
The functional groups in a macromolecule are usually attached to the carbon backbone at one or several different places along its chain and/or ring structure. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
A functional group can participate in specific chemical reactions. Some of the important functional groups in biological molecules are shown in Figure 2.30; they include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl. These groups play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids. Functional groups are usually classified as hydrophobic or hydrophilic depending on their charge or polarity characteristics. An example of a hydrophobic group is the non-polar methane molecule. Among the hydrophilic functional groups is the carboxyl group found in amino acids, some amino acid side chains, and the fatty acids that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions (H+) from the COOH group resulting in the negatively charged COO- group; this contributes to the hydrophilic nature of whatever molecule it is found on. Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic.
Hydrogen bonds between functional groups, within the same molecule or between different molecules, are important to the function of many macromolecules and help them to fold properly into and maintain the appropriate shape for functioning. Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing and the binding of an enzyme to its substrate, as illustrated in Figure 2.31.
Science Practice Connection for AP® Courses
Activity
Carbon forms the backbone of important biological molecules. Create a mini-poster of a simple food chain that shows how carbon enters and exits each organism on the chain. Based on the food chain you created, make a prediction regarding the impact of human activity on the supply of carbon in the food chain.